How often do we hear about the 100 year design life, e.g. for a bridge, yet major structural repairs are required even before the structure is 30 years old and reaching 100 years, without huge expenditure on repairs way in excess of the original cost of the structure, is probably just a dream.
Annually, billions of pounds are spent globally repairing/replacing concrete structures. Much of this could be saved and the money more productively used had those structures been built differently.
The reasons for this are many but here are three to consider. First, bids for building the structure come in higher than expected or over budget. To reduce costs, cheaper options are proposed including those for corrosion protection to concrete. Second, ‘fast track’ schedules and penalties for late completion compromise quality as corners are cut, leading to defects that will ultimately require expensive repair. Third, untried or inappropriate products are used which subsequently fail to perform as promised in the environment used. This might occur as a result of lack of knowledge or false assumptions. The use of high-alumina cement and aggregates containing pyrite and calcined dolomite spring to mind.
Where concrete structures are built in potentially corrosive environments, owners should be extremely wary of persuasion to use alternative products to those specified by the structural engineer, especially motivated by price. “There is hardly anything in the world that some man can’t make just a little worse and sell just a little cheaper, and the people who buy on price alone are this man’s lawful prey” – John Ruskin.
For years there has been discussion in the construction industry about “whole life costing” but in practice this has been little more than bar-room chat and those few efforts that have been made have been over-ridden by the issue of up-front capital cost.
 There are so many examples to choose from, especially bridges and tunnels. The Öland Bridge repairs completed in 2005 after about 15 years in service and involving renovation work to the corroded pier supports in the tidal zone, replacement of 19 joints and restoration of the parapet walls cost: “… twice the (total) cost of the original bridge at current prices”. The project manager is quoted as saying: “Buy cheap, repair expensively”.
There are so many examples to choose from, especially bridges and tunnels. The Öland Bridge repairs completed in 2005 after about 15 years in service and involving renovation work to the corroded pier supports in the tidal zone, replacement of 19 joints and restoration of the parapet walls cost: “… twice the (total) cost of the original bridge at current prices”. The project manager is quoted as saying: “Buy cheap, repair expensively”.
If we are serious about ‘sustainability’, we need to address this issue and make concrete perform better. We may not be able to completely stop concrete structures deteriorating but we can significantly extend the life of such structures with proven products which are available here and now.
Concrete can deteriorate for a variety of reasons, and concrete damage is often the result of a combination of factors.
Chloride induced corrosion to reinforcement and other embedded metals is the leading cause of deterioration in concrete. When steel corrodes, the resulting rust occupies a greater volume than the steel. The resultant expansion creates tensile stresses in the concrete, which can eventually cause cracking, delamination, and spalling. Sulphates attack concrete by reacting with hydrated compounds in the hardened cement. These reactions can induce sufficient pressure to disrupt the cement paste, resulting in loss of cohesion and strength.
ASR (Alkali Silica Attack), Freeze thaw damage, Acid Attack, Carbonation are also well known forms of concrete deterioration and premature failure.
In reinforced concrete, all common forms of serious deterioration are as a result of water ingress. If concrete could be kept inherently dry, most corrosion issues would disappear. However, it is very important to understand the mechanisms by which water passes through concrete. Some mistakenly assume that ‘low permeability’ is the key parameter. The most common test for permeability simply measures concrete density as water at pressure is applied to the surface. The so-called ‘permeability’ is determined by the depth of ingress, which in turn, is determined by the density of the concrete. Permeability, by definition, is a measure of flow under an external pressure and is a property of saturated materials. Ordinary good quality concrete resists any appreciable flow of water under pressure, making the concrete stronger or denser is completely irrelevant. By increasing density, far from slowing the passage of water, you may actually speed up the flow by capillarity. The predominant mechanism of water movement through concrete, simple capillary absorption, requires no hydrostatic pressure whatsoever – the narrower the pores in saturated concrete, the lower its permeability. Conversely, the narrower the pores, the greater the resultant capillary pressure and so the greater the depth and speed of water ingress. “Calculation of the water penetration depth during wetting showed that the speed of capillary absorption is of the order of a million times faster than permeability” *1.
Experience around the world over the last 50 years has clearly demonstrated that ordinary reinforced concrete used in marine environments is highly susceptible to corrosion.
Field experience and advanced research shows and confirms that, in air-exposed marine structures such as those in tunnels or submerged basements, capillary absorption is the primary water and chloride transport mechanism. The idea of overcoming corrosion problems by using higher strength or more dense concrete with lower permeability has been shown to be counter-productive and to have worsened the problem.
 The root cause of chloride induced concrete corrosion is the fact that all normal concrete is very absorptive and the speed of absorption is rapid. Under conditions of wetting and drying it relentlessly sucks in and absorbs water, moisture and contained salts.
The root cause of chloride induced concrete corrosion is the fact that all normal concrete is very absorptive and the speed of absorption is rapid. Under conditions of wetting and drying it relentlessly sucks in and absorbs water, moisture and contained salts.
In a tunnel with moving traffic and ventilation there will be a constant movement of warm air. As the absorbed sea water reaches the inner surface of the tunnel, or basement, it will quickly evaporate allowing more water to be absorbed such that there will be a constant flow of water from the external to the internal side.
However, the salt carried in the water does not evaporate and is left behind and will continually build up until the concentration at the inner face reinforcement is enough to destroy the high alkalinity at the concrete to rebar interface. Now all that is required for the steel to start corroding is oxygen (available within the basement) and an electrolyte (water).
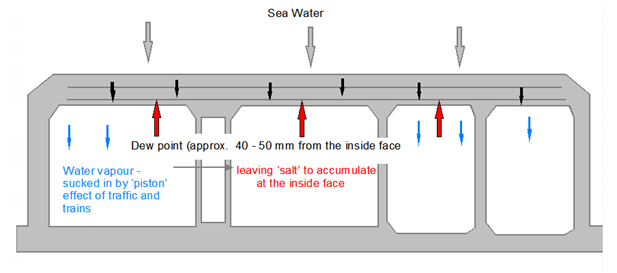
It is worth noting that corrosion reactivity can be expected to double for every 100C rise in temperature.
The challenge therefore is how to fully and effectively protect sub-sea air exposed concrete structures or reinforced concrete in the tidal or spray zone from chloride induced corrosion.
Since the reality of chloride-induced corrosion of reinforced concrete became apparent, owners, engineers and designers have applied a wide variety of methods to reduce water and chloride penetrability of concrete, and so delay or try to prevent corrosion.
Most of these have focused on “densification” using higher strength and/or supplementary cementitious materials, such as silica fume or blast furnace slag, with the aim of reducing concrete permeability and related chloride diffusion. All have practical limitations, while none address the problem of capillary absorption.
Increasing compressive strength and/or cement contents. Mehta & Burrows observe; “..The belief that the higher the strength of concrete, the more durable will be the structure, is not supported by field experience. High‑early strength concrete mixtures are more crack‑prone and deteriorate faster in corrosive environments. Codes should be amended to stress this point adequately”.*4
Increasing the cement content above the structure’s requirements could actually add to the corrosion problem. Beyond an optimum amount, more cement does not usefully decrease the volume of pores and capillaries, but does exacerbate thermal and micro-cracking and autogenous shrinkage, and therefore the rate of salt-water absorption.
Increasing concrete cover to steel. In the absence of defect-free coatings or other barriers, the concrete cover and its penetrability characteristics are the only protection provided to embedded reinforcement from the moment of casting and throughout the service-life. Yet the requirements for greater cover are based on chloride diffusion models which assume saturated conditions, no absorption and uncracked concrete. None of these conditions exist in-service. In practice, their opposites do.
As a result, concrete cover to steel has been increased in many countries. In the USA, up to 100mm is in use, yet without anything like a corresponding increase in “time-to-corrosion” (Florida’s 100mm cover in bridge structures). Aside from dramatically increasing the weight, cost and microcracking of the structures, it is ultimately inadequate, as the presence of cracking and the rate of water absorption negates the additional cover. At each cracked location in absorptive concrete, protection of the reinforcement is absent.
Silica fume. Silica fume provides higher compressive strength for little cost and favourably modifies diffusion if fully water cured. But it has an insignificant effect on water absorption, so it absorbs and loses water freely. Without full curing, or in the presence of microcracking, silica fume can actually increase absorption. Silica fume’s tendency to increase the brittleness of concrete increases its tendency toward cracking / microcracking. This risk is increased by “stickiness” / workability problems at higher placement temperatures, requiring excessive batch water that is not always compensated for by superplasticisers.
Blast furnace slag cements. Concretes made with slag cements can provide useful reductions in heat of hydration as well as chloride diffusivity, and its use is recommended where practical. However slag does not have any significant beneficial effect on absorption, so like silica fume, it remains ultimately vulnerable to absorption/desorption and the same corrosion processes as concrete without it, including at cracks.
Use of ‘inhibitor’ additives. Nitrites require the maintenance of a ratio of nitrite:chloride in excess of 1.5:1 to have effect. Initial exposure to absorbed chlorides shows inhibition of anodic activity. However, in practice the inhibitor is water soluble and is always dissolved and drawn away to an evaporating surface by absorbed water transpiring through the concrete, particularly at cracks. The reducing nitrite:chloride ratio of 1.5:1 is overwhelmed by constantly increasing levels of absorbed chlorides. Steel corrosion then occurs at the usual rates – some suggest at increased rates, without any hindrance or inhibition.
Cathodic Protection or “CP”. CP involves electrically connecting all steel reinforcing bars and wiring the whole setup to a monitor that detects the presence and amount of electrical corrosive current in the unit, and then generates an equal, opposite current in an effort to neutralise the corrosive current. CP does not have any effect on any other corrosion mechanisms, so the cement paste is still vulnerable, as are any other embedded metals in the structure, such as lighting posts, rails, etc., that are not linked to the CP ‘battery’. CP is expensive to install and of course is maintenance intensive, in terms of monitoring currents generated (If excessive currents are generated, corrosion is obviously accelerated).
CP is rendered ineffective where connections are broken, faulty or not secure and naturally, as the potential of corrosive currents vary over the structure, so the counter-current generated will never be exactly equal. Therefore, some corrosion current always exists, and so does corrosion.
Use of impregnating water repellents. Despite the practical problems of application, the use of post-construction surface-applied impregnations, such as silanes, siloxanes, etc., are helpful, especially for protection of pre-cast elements if sea-barged at early-age when they are particularly vulnerable to chloride contamination (see “c” below). By creating a hydrophobic zone in the outer millimetre or two of the all-important concrete cover, silanes usefully improve its saltwater-resistance, or at least that portion of it. However, a number of considerable limitations and practical problems remain for the user:
- Higher strength, denser concretes do not absorb silanes past the say, 1 to 2mm mark, leaving them at the surface and therefore limiting their effectiveness.
- It is impractical to apply a liquid curing membrane (pre-cast or in-situ) as it will impair the later application of the silane. Extended water curing is necessary.
- For proper “binding”, silanes should be applied after the concrete has cured and been allowed to dry to a proscribed internal humidity. During this time unprotected, immature concrete is highly vulnerable to salt water absorption and contamination – Research by Taywood shows that owing to the effects of absorption in early-age / immature marine concrete, up to 50% of the chloride required for activation of steel enters the concrete within the first 3 months exposure*5. Silane treatment could help protect “young” (un-cracked) pre-cast elements against salt-contamination during floating to the location site, but would also require yard-storage and double-handling prior to shipment, and would likely incur significant costs.
- Silanes depend upon 100% correct and complete coverage of ALL exposed concrete surfaces, otherwise water is naturally absorbed through any non-treated areas and corrosion can begin. Access to some areas may be too difficult to allow coverage at all.
- Cracks and mechanical surface damage that occur in concrete after silane application provide a pathway past the silane treated surface, and instant / immediate access to the concrete interior. The underlying concrete again begins to corrode as salt water is absorbed via these defects to the interior and the steel.
- Silane manufacturers state that re-application of silanes should occur every ten years to maintain effectiveness. In addition to the environmental constraints of the site, this maintenance requirement brings into play the same negating factors above, let alone the ongoing expense.
Everdure Caltite the extra ingredient for proven durability
Cast iron is not used in modern construction because it has a critical “deficiency”. It is brittle. However, adding a small amount of carbon (0.2%) gives us high tensile strength – “steel”. For additional enhancement adding chrome gives “stainless” steel and so on. Everdure Caltite applies the same proven concept to concrete.
Everdure Caltite is a hydrophobic and pore-blocking liquid ingredient which includes a corrosion inhibitor and is added to the concrete mix. It reverses normal capillarity (sucking action) to produce ultra-low absorption concrete.
Normal high quality concrete is unsuitable for marine structures. It naturally absorbs water, moisture, and any deleterious salts in solution and provides the all important electrolyte linking anodic and cathodic regions of the reinforcement*2.

Adding another ingredient, Everdure Caltite, to normal concrete effectively transforms this deficiency, giving it properties with significant technical, commercial and environmental advantages.
In 2004 at Dundee University, UK *3, high-quality concrete segments made with and without Caltite were subjected to sustained dynamic load and severe corrosion conditions by long-term salt water wet/dry cycling in a specially constructed chamber (4 x Wet/Dry cycles per day, over 16months equivalent to 52 Years marine exposure). The tests showed that the inclusion of Caltite delayed initiation of reinforcement corrosion activity by a factor of 50 times. This is consistent with existing empirical evidence of Caltite used in marine concrete.
 Seabird Naval Base Karwar, India
Seabird Naval Base Karwar, India
Concrete containing Everdure Caltite was used in various parts of the structure to provide waterproofing and corrosion protection.
*1 ‘Capillary absorption by concrete’ (Concrete July/August 1997) Dr Andrew Butler, Transport Research Laboratory
*2 The photos show concrete supports for a pipeline that crosses a tidal salt pan in South Australia where summer temperatures can reach 40°C. To put this into perspective, the ground sulfate levels on this site were measured at 7.2g/litre and chlorides at 53g/litre. These exceed the worst possible cases indicated in BRE Special Digest 1 – (DS5 & DC4).
*3 Corrosion resistance of concrete structurss modified with Everdure Caltite System, Dundee University, Scotland, December 2004, Dr Chun Q Li.
*4 Building Durable Structures in the 20th Century, American Concrete Institute Magazine, March 2001, P.K. Metha / R. Burrows
